Where Are Alkaline Earth Metals Found On The Periodic Table Group 1 Group 2 Groups 3 12 Group 17
Camila Farah
Coauthor of inorganic chemistry.
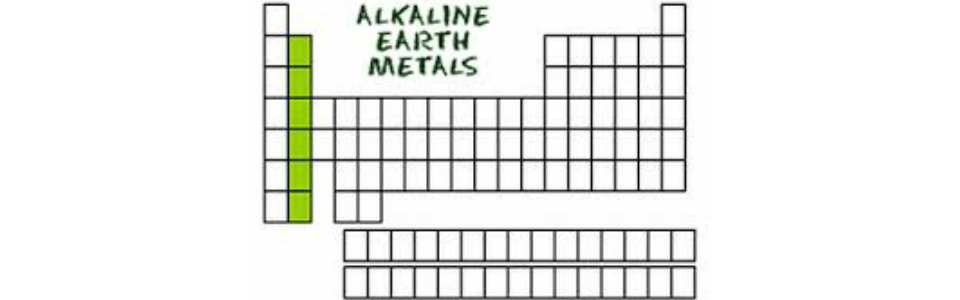
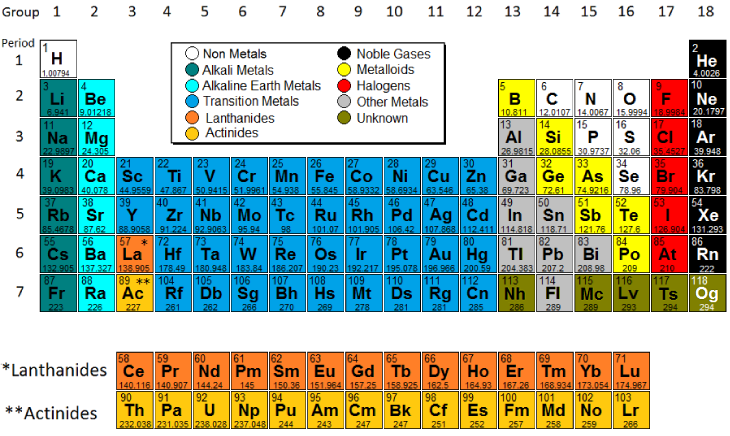
Structurally they have in common an outer s orbital which is full. They are all shiny silvery white somewhat reactive metals at standard temperature and pressure. Atoms of metallic elements tend to. Alkaline earth metals are also known as group 2 elements.
Lose electrons and form positive ions. The alkaline earth metals are six chemical elements in group 2 of the periodic table. Modern version of the periodic table of the elements. Alkaline earth metal any of the six chemical elements that comprise group 2 iia of the periodic table.
They are placed in the vertical column on the left hand side of the periodic table. They must be stored under oil to keep air and water away from them. They are beryllium magnesium calcium strontium barium and radium. Alkali metal any of the six chemical elements that make up group 1 ia of the periodic table namely lithium li sodium na potassium k rubidium rb cesium cs and francium fr.
RELATED ARTICLE :
- pink and white ombre nails with diamonds
- pink and gold minnie mouse party supplies
- pin the tail on the donkey game
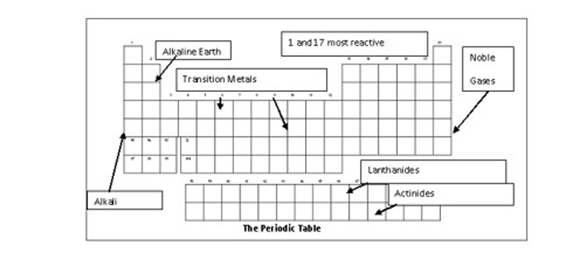
Groups in the periodic table of elements click on an element to read about the chemical and physical properties of the group to which that element belongs. The alkali metals consist of the chemical elements lithium li sodium na potassium k rubidium rb caesium cs and francium fr. Together with hydrogen they constitute group 1 which lies in the s block of the periodic table. Alkali alkaline earth metals and halogens are found in groups.
Elements of this group are beryllium magnesium calcium strontium barium and radium. Thus we can conclude that out of the given options alkaline earth metals are found on the periodic table in group 2. The elements have very similar properties. Metals outnumber nonmetals on the periodic table because most of the elements.
Where are alkaline earth metals found on the periodic table. Alkali metals include lithium sodium potassium rubidium and cesium. Elements of group 1 are called alkali metals and elements of group 17 are called halogens. Group 1 group 2 groups 3 12 group 17.
RELATED ARTICLE :
You ll find more specific groups like transition metals rare earths alkali metals alkaline earth halogens and noble gasses.Source : pinterest.com